
Yes, hydrogen bonding occur between two nh3 molecules because if you look at the structure of nh3, hydrogen are directly attached with nitrogen (high electronegative atom). Let’s know in details about intermolecular forces such as, (hydrogen bonding and dipole dipole intraction, london dispersion forces). this types of intermolecular forces are generated between nh3 molecules. In case of nh3, (N-H bonds makes between molecules) and dipole dipole interaction (interaction between two dipole) and london dispersion forces occur between nh3 molecules. Yes, it is true, hydrogen bonding is strongest intermolecular forces compare with all. There are three main major intermolecular forces occur between nh3 molecules such as, Lets know in details, how this type of interaction occur between nh3 molecules. nh3 molecules generate three different type of intermolecular forces, Such as, hydrogen bonding, dipole dipole intraction and london dispersion forces. this type of intermolecular forces are occur between nh3 molecules. nh3 (ammonia) molecules has three different intermolecular forces, such as, hydrogen bonding and dipole-dipole intraction and london dispersion forces. As a result, hydrogen bonding and dipole-dipole and london dispersion forces are generate between molecules.

and due to this electronegativity difference between nitrogen and hydrogen, partial negative charge appear on nitrogen and partial positive charge appear on hydrogen.

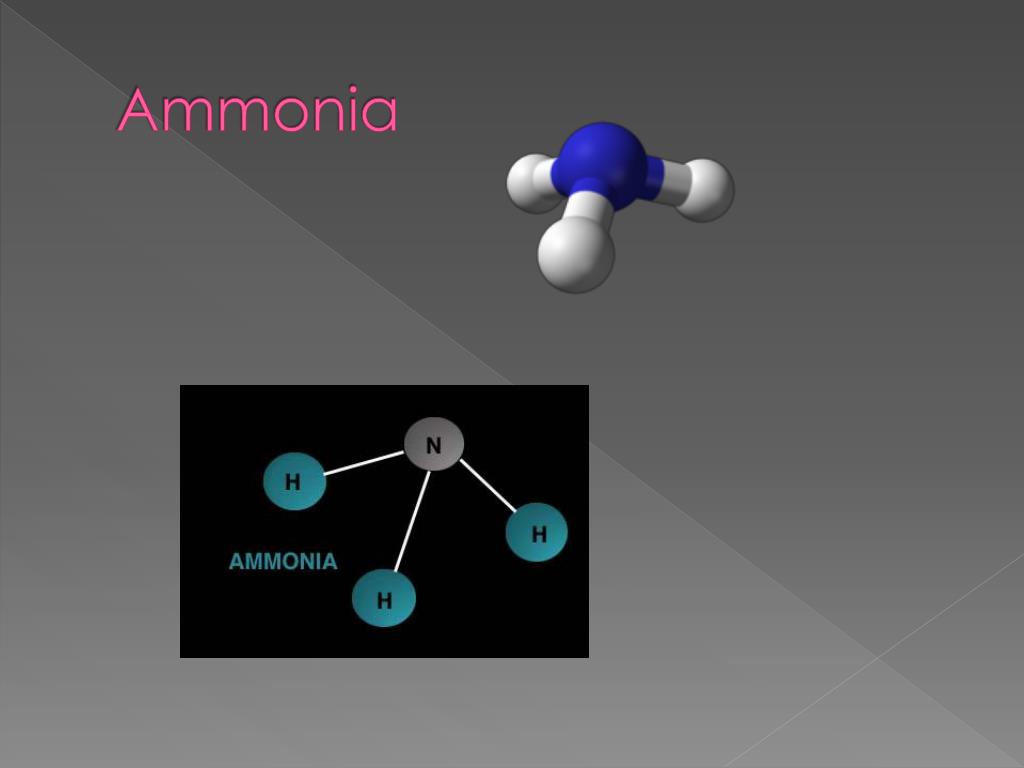
due to this, or As a results hydrogen bonding occur between them. so, large difference of electronegativity between nitrogen and hydrogen. but nitrogen has highly electronegative value. In this molecules, hydrogen are directly connected with nitrogen. It make N-H bonds due to hydrogen are directly attached with nitrogen. it genrate hydrogen bonding and dipole dipole intraction. The Intermolecular forces are occur between nh3 (ammonia) molecules. What are the intermolecular forces of NH3? Intermolecular intermolecular forces occur between two polarized molecules. it attract between partial negative end of one molecules to partial positive end of another molecules. this type of forces are called intermolecular forces. this forces are also mediate force of attraction and repulsion between molecules of a substance. Intermolecular forces are the forces which mediate attraction between molecules in a substance. Lets get started, What is intermolecular forces? So, read complete article, you got better knowledge regarding this topic. So, hold your seat end of out, because we will provide valuable information regarding this topic. Also covered about, different types of intermolecular forces, polarity and FAQ. here, we will discuss about, what is the intermolecular forces of nh3 molecules. Place the remaining two electrons on the nitrogen atom.Hello, reders welcome to another fresh article. Now draw a third hydrogen atom next to the nitrogen atom and place a third pair of electrons between these atoms.

Now draw a second hydrogen atom next to the nitrogen atom and place a second pair of electrons between these atoms. The total number of electrons for ammonia will therefore be 8 electrons.ĭraw a hydrogen atom next to a nitrogen atom and place a pair of electrons between these two atoms. The nitrogen atom has 5 electrons and each of the three hydrogen atoms has 1 electron. What are the numbers of bonding electrons for the nitrogen atom and the hydrogen atoms? The arabic numeral above the element's column in the periodic table gives you that number. The first step in drawing the electron dot formula for ammonia is to determine the number of bonding electrons for each of the atoms. What is the first step that you must do in order to draw the electron dot formula for ammonia, NH 3?
If you get stuck, try asking another group for help. You should try to answer the questions without referring to your textbook.


 0 kommentar(er)
0 kommentar(er)
